
At AIP Precision Machining, we understand that plastic machining has fundamentally transformed the manufacturing industry, enabling unprecedented precision and versatility in creating custom components. Leveraging over four decades of experience, we combine cutting-edge technology with an in-depth understanding of material properties to deliver high-quality parts across various applications. From aerospace to medical devices, our expertise in plastic machining plays a pivotal role in shaping the products that industries rely on daily. This requires advanced techniques such as CNC machining, drilling, and milling and a deep commitment to precision and innovation.
As leaders in the machining of high-performance plastics and composites, AIP is dedicated to pushing the boundaries of what is possible with materials like PEEK. Our advanced capabilities ensure that every component we produce meets the stringent demands of modern applications. Through meticulous attention to factors such as thermal expansion and dimensional stability, we deliver parts that are both reliable and exact in their specifications.
In this guide, we provide insights into the intricacies of plastic machining, sharing our knowledge on selecting appropriate materials and the critical factors contributing to successful outcomes.
We cover specialized machining processes, quality control measures, and surface finishing techniques—key elements that, when mastered, enable manufacturers to optimize their operations and achieve superior results.
At AIP, our mission is to ensure that every project we undertake meets and exceeds industry standards, delivering precision fabrication and unmatched quality.
key takeaways
| Section | Key Takeaway |
| Understanding Plastic Materials | Selecting the right plastic material, like PEEK or Torlon, is crucial for optimal machining outcomes. |
| Mechanical Properties | Mechanical strength, such as tensile and impact resistance, is critical in determining material suitability for specific applications. |
| Thermal Properties | Thermal expansion and heat deflection are key factors affecting machining precision and component stability. |
| Chemical Resistance | Materials like PEEK and PTFE offer superior chemical resistance, making them ideal for harsh environments. |
| Specialized Applications | Engineering plastics are essential for high-performance applications in industries like aerospace and medical devices. |
| Quality Control and Finishing | Rigorous quality control processes ensure that machined plastic components meet exact specifications and industry standards. |
Understanding Plastic Materials for Machining
At AIP Precision Machining, we recognize that plastic machining has become a vital part of modern manufacturing, offering unmatched versatility and precision.
Achieving optimal results in this field requires a deep understanding of the materials used. Each plastic, whether PEEK, PTFE, or Torlon™™, has unique properties such as thermal stability, chemical resistance, and mechanical strength that must be carefully considered.
Selecting a suitable material is crucial, directly impacting machining techniques and final product quality. At AIP, our extensive experience ensures that we guide our clients in choosing the best materials for their specific applications. By mastering these material properties, we deliver precision and reliability in every project.
Types of Machinable Plastics
Machinable plastics are broadly classified into two categories: thermoplastics and thermosets. The larger category, thermoplastics, can be softened and melted multiple times without chemical change. They exhibit high creep resistance and solubility in certain solvents and can be enhanced with fillers like carbon fibers or glass fibers. Common thermoplastics include ABS, acetal, acrylic, nylon, polyethylene, and polypropylene.
Once cured, thermosets, however, do not melt or soften under heat. They are insoluble in most solvents, have high creep resistance, and are susceptible to chipping. Examples of thermosets include Bakelite, epoxy, and phenolics.
Material Properties and Considerations
When selecting a plastic for machining, two primary factors come into play: the material’s suitability for the application and its machinability. Fundamental properties to consider include:
- Biocompatibility
- Chemical resistance
- Dimensional stability
- FDA classification
- Impact resistance
- Loading strength
- Temperature resistance
- UV resistance
- Wear resistance
Thermal Characteristics
The thermal properties of plastics significantly impact the machining process. Plastics have a thermal expansion rate up to 20 times greater than metals and lose heat more slowly, increasing the risk of localized overheating. Their softening and melting temperatures are much lower than metals, requiring careful consideration during machining operations.
| Property | Plastic vs. Metal |
| Thermal Expansion | Up to 20 times greater |
| Heat Dissipation | Slower |
| Melting Temperature | Much lower |
Proper heat management is crucial in plastic machining to prevent dimensional shifts and stress cracking. Choosing appropriate cutting tools and managing mill speeds are also essential for maintaining workpiece integrity and achieving desired results in CNC plastic machining.
Essential Plastic Machining Techniques
Plastic machining techniques have revolutionized manufacturing, offering precision and versatility in creating custom components. These methods require a deep understanding of material properties and specialized approaches to ensure optimal results.
CNC Milling
CNC milling has become a cornerstone of plastic machining. This technique employs a high-speed cylindrical cutting tool to subtract material from a stationary plastic shape. The computerized control enhances accuracy and efficiency, allowing for complex geometries and tight tolerances. Properly stabilizing the workpiece is crucial when milling plastics to minimize vibrations and prevent chatter marks. Climb milling, also known as down milling, is generally recommended for superior results.
Turning
Turning involves rotating a plastic shape around a stationary lathe, making it ideal for symmetrical parts. Heat management is critical in this process due to the thermal properties of plastics. Operators must carefully consider rotation speed, tool selection, and coolants to prevent damage. Inserts with positive geometries and ground peripheries are preferred, often utilizing fine-grained C-2 carbide for optimal performance.
Drilling
Drilling in plastics requires special attention to heat dissipation, particularly for deep holes. High-speed steel twist drills suffice for small diameter holes (1/32″ to 1″). Larger holes demand slower speeds and may require pilot holes. Peck drilling is recommended to improve swarf removal and prevent heat buildup. A slow spiral drill or a general-purpose bit ground to a 118° point angle with 9° to 15° lip clearance is ideal for larger diameters.
Cutting and Sawing
Sawing plastics differs significantly from metal cutting due to the lower softening and melting temperatures of plastics. Band saws excel in making straight and curved cuts, while table saws are preferred for straight cuts in thicker materials. Blade selection is crucial; rip and combination blades with a 0° tooth rake and a 3° to 10° tooth set are optimal for general sawing. Tungsten carbide blades provide superior wear resistance and surface finish.
Quality Control and Finishing in Plastic Machining
At AIP Precision, quality control and finishing are critical to delivering precision-engineered plastic components. Certified to AS 9100, ISO 13485, and ISO 9001 standards, we adhere to stringent protocols ensuring every part meets specifications.
Our quality control process involves detailed inspection and defect detection at each production stage, utilizing advanced metrology tools to maintain tight tolerances. Surface finishing techniques are then applied to enhance performance, ensuring that each component is accurate and meets the highest standards of durability and functionality.
In the following sections, we’ll outline our approach to quality control and finishing and detail the methods that drive consistent, high-quality results in plastic machining.
Inspection Techniques
Plastic parts inspection employs various methods to assess quality and conformity. Visual checks, often aided by magnifiers and microscopes, serve as the first line of defense against surface imperfections. Dimensional inspection utilizes tools like Coordinate Measuring Machines (CMM) to ensure precise adherence to specifications. Functional testing assesses performance requirements, while material testing confirms desired properties such as strength and durability.
Advanced inspection techniques include:
- 3D scanning: Creates detailed models for comprehensive analysis
- Ultrasonic inspection: Identifies internal defects without altering the part
- X-ray and CT scanning: Reveals internal structures non-destructively
- Acoustic emission testing: Analyzes sound to detect inconsistencies
Common Defects and Solutions
Plastic machining can result in various defects that impact product quality. Some common issues include:
| Defect | Cause | Solution |
| Flow lines | Uneven cooling | Adjust injection speed and temperature |
| Sink marks | Thick sections | Optimize part design and cooling process |
| Warping | Uneven internal shrinkage | Ensure uniform cooling |
| Delamination | Contaminants | Control material quality and process parameters |
| Short shots | Restricted flow | Adjust injection pressure and gate design |
Addressing these defects requires a combination of process optimization, material selection, and design modifications. Early detection through rigorous inspection helps minimize waste and rework.
Surface Finishing Methods
Surface finishing enhances both the aesthetics and functionality of plastic parts. Common techniques include:
- Graining: Achieves a Ra of 4 to 32
- Lapping: Produces a finer finish with Ra of 2 to 16
- Wet sanding: Progressively applies finer grits for a smooth surface
- Flame polishing: Applies heat to level out uneven spots
These methods improve appearance and serve practical purposes, such as hiding tooling marks and improving paint adhesion. The finishing technique chosen depends on the part’s specific requirements and intended application.
Selecting the Right Plastic for Machining Projects
Engineering Plastics vs Commodity Plastics
Selecting the appropriate plastic for machining projects is a critical step that demands a deep understanding of material properties and application requirements. At AIP Precision Machining, we specialize in machining high-performance engineering plastics, such as Torlon™, PEEK, and Ultem®, which are distinct from commodity plastics in their capability to withstand extreme conditions.
Commodity Plastics are commonly found in everyday household items and are produced in large volumes at relatively low costs. These materials are typically designed for single-use products, offering sufficient durability for their intended purpose but lacking the advanced properties needed for more demanding applications. Commodity plastics include polyethylene (PE) and polypropylene (PP), ideal for products where cost efficiency and disposability are prioritized.
In contrast, Engineering Plastics like Torlon™ (PAI), PEEK, and Ultem® (PEI) are formulated to perform under harsh chemical, mechanical, and environmental conditions. These materials are produced in smaller quantities and are selected for applications where strength, thermal stability, and chemical resistance are paramount.
Torlon™ (Polyamide-Imide, PAI) is renowned for its high mechanical strength and outstanding wear resistance, even at elevated temperatures up to 500°F (260°C). This makes it ideal for aerospace components, bearing cages, and high-temperature electrical connectors.
PEEK (Polyetheretherketone) offers exceptional chemical resistance and can be used continuously at temperatures up to 480°F (250°C). Its high strength-to-weight ratio and ability to withstand harsh environments make it a preferred material for aerospace, medical implants, and semiconductor manufacturing equipment.
Ultem® (Polyetherimide, PEI) is valued for its excellent flame resistance, high dielectric strength, and resistance to heat and chemicals. It is commonly used in applications requiring high rigidity and stability, such as electronic insulators, reusable medical devices, and structural components in demanding environments.
When selecting a suitable plastic for a machining project, it’s essential to match the material’s properties to the specific demands of the application. Engineering plastics like Torlon™, PEEK, and Ultem® are chosen for their ability to withstand stress and harsh conditions and for their machinability, ensuring that the final product meets the exacting standards required in critical industries.
Material Properties Comparison
When comparing materials, it’s crucial to consider various properties:
- Tensile Strength: Engineering plastics like PEEK (14,000 psi) and PAI (21,000 psi) offer superior tensile strength compared to commodity plastics.
- Flexural Modulus: Glass-filled materials such as Ultem®® (1,300,000 psi) provide exceptional stiffness.
- Impact Resistance: Some commodity plastics like LDPE have high impact resistance, while engineering plastics like PEEK (6.1 ft-lbs/in) offer a balance of strength and toughness.
- Temperature Resistance: Engineering plastics generally have higher heat deflection temperatures, making them suitable for high-temperature applications.
Application-specific Material Selection
To select the suitable plastic for a specific application:
- Define requirements: Consider factors such as load-bearing capacity, temperature exposure, chemical resistance, and dimensional stability.
- Evaluate properties: Analyze tensile strength, flexural modulus, impact resistance, and dielectric strength of potential materials.
- Consider regulatory requirements: Check if FDA compliance or specific UL94 flame retardant ratings are necessary.
- Test candidates: After narrowing down options, conduct thorough testing to ensure fitness for use in the specific application.
By carefully considering these factors, manufacturers can choose the most suitable plastic material for their machining projects, ensuring optimal performance and longevity.
Ensuring Dimensional Stability in Machined Plastic Parts
Maintaining dimensional stability in machined plastic parts is crucial for ensuring optimal performance and longevity. This process involves addressing internal stresses, controlling moisture, and managing temperature fluctuations. By implementing proper techniques, manufacturers can achieve tighter tolerances and enhance the overall quality of plastic components.
Stress Relief and Annealing
Internal stresses in plastic parts can lead to warping, twisting, and dimensional changes. These stresses often result from the manufacturing process or machining operations. To mitigate these issues, annealing has become an essential technique. This process involves slowly heating the polymer part to a temperature just below its softening point, holding it there for a specified time, and then allowing it to cool gradually. Annealing offers several benefits:
- Improved dimensional stability
- Enhanced flatness
- Better chemical resistance in clear plastics
- Increased wear resistance in materials like Torlon™
For high-performance plastics, annealing may occur on raw materials before machining or on finished parts, depending on the material properties and application requirements.
Moisture Control
Moisture absorption can significantly impact the dimensional stability of plastic parts. Many polymers are hygroscopic, meaning they absorb moisture from their environment. This absorption can lead to:
- Dimensional changes
- Reduced strength
- Altered glass transition temperature
Proper storage and packaging are essential to address these issues. Vacuum-sealing parts in moisture-impermeable layers or using desiccant bags can prevent moisture uptake in humid environments. Additionally, drying parts before use or reuse may be necessary for critical applications.
Temperature Considerations
Temperature plays a crucial role in maintaining dimensional stability. Plastic materials have lower melting points than metals, requiring effective thermal regulation during machining. Key temperature-related factors include:
Heat Deflection Temperature (HDT)
Glass Transition Temperature (Tg)
Continuous Use Temperature (CUT)
Proper cooling techniques, such as using water-based coolants, help manage heat generation during machining. This is particularly important for materials like ABS, polycarbonate, and PTFE, which are sensitive to temperature fluctuations.
By addressing these factors, manufacturers can ensure the production of high-quality, dimensionally stable plastic parts that meet stringent performance requirements across various industries.
Specialized Plastic Machining Applications
At AIP, we understand that specialized industries require materials and machining processes beyond the ordinary. In medical device manufacturing, aerospace, power and energy, and specialized industrial applications, the need for high-performance, precision-engineered plastic components is paramount. These industries rely on materials that offer superior mechanical strength, chemical resistance, and the ability to withstand extreme conditions—all while maintaining tight tolerances and lightweight profiles.
In this section, we explore how plastic machining meets the rigorous demands of these specialized applications. From producing critical medical devices to manufacturing high-strength aerospace components and from power and energy solutions to complex industrial parts, our expertise in machining advanced materials like PEEK, Torlon, and Ultem ensures that every component meets and exceeds industry standards. By leveraging our knowledge and state-of-the-art technology, we deliver precision-engineered solutions that drive innovation and efficiency across these vital sectors.
Medical Device Manufacturing
Plastic machining has revolutionized the medical industry, offering versatile solutions for high-performing medical devices. The fabrication of these components is critical to providing quality healthcare services. Companies like PlasTech Machining & Fabrication specialize in creating high-grade medical devices using various materials such as acrylics, polymers, polycarbonate, and PVC. These materials have made healthcare safer, cost-effective, and more efficient. Plastics are widely used in creating medical safety devices like blister packs and tamper-proof caps, as well as non-permeable bags for storage and transportation of medical items.
Aerospace Components
The aerospace industry relies heavily on lightweight, tight-tolerance components that maintain mechanical strength under extreme conditions. CNC machining is the preferred method for achieving the precision needed in aerospace applications. This process allows for the conversion of metal components to high-performance and engineering plastics, resulting in significant weight reduction and fuel efficiency improvements. Materials such as PEEK, PPSU, PI, and PEI are commonly used in aerospace applications due to their exceptional properties, including high strength-to-weight ratios, temperature resistance, and chemical resistance.
Power and Energy Components
High-performance plastic components have transformed the power and energy industry due to their lightweight design and ability to withstand hostile conditions. Materials like PEEK, PVDF, and PPS offer unique blends of electrical, wear, chemical, and temperature resistance, making them ideal for oil and gas industry applications. These plastics drive advancements in oil-drilling and distribution, as well as provide solutions for exploration and development in offshore reserves. Typical applications include connector bodies, sealing systems, valve components, and wear products such as bearings and yoke bushings.
Specialized Industrial Parts
Plastic machining caters to various specialized industrial applications, offering custom solutions for complex requirements. High-performance plastics like TORLON™ (PAI) are ideal for critical mechanical and wear components in turbine engines, automotive transmissions, and heavy-duty equipment. These materials provide reliable performance under severe temperature and stress conditions. Applications include bearing cages, high-temperature electrical connectors, structural parts, valve seats, and wear rings. The versatility of plastic machining allows for the creation of specialized parts that meet specific industry needs while offering superior performance and longevity.
Fundamentals of Plastic Material Properties
Understanding the fundamental properties of plastic materials is essential for optimizing their performance in demanding applications. At AIP, we prioritize these properties—mechanical strength, thermal stability, and chemical resistance—when selecting and machining materials like PEEK, PTFE, and Acetal.
This section explores how these key properties influence material choice and machining processes, ensuring that each component meets the stringent requirements of its intended application. Whether it’s the tensile strength needed in aerospace or the chemical resistance required in industrial settings, mastering these fundamentals is crucial to delivering reliable, high-performance plastic parts.
Mechanical Properties
Plastic materials exhibit diverse mechanical characteristics crucial for their performance in various applications. Tensile strength, impact resistance, and wear resistance are key factors in material selection. For instance, PEEK offers exceptional mechanical resistance, making it ideal for demanding aerospace and automotive applications. Acetal, known for its dimensional stability, maintains integrity under repeated impacts and temperature variations, suiting it for high-stress industrial uses.
Thermal Properties
Thermal properties significantly influence plastic behavior during machining and end-use. The coefficient of thermal expansion for plastics is typically higher than metals, leading to more substantial dimensional changes during heating. Heat Deflection Temperature (HDT) indicates when a material begins to deform under elevated temperatures, a critical consideration for machining processes and final applications. Some plastics, like PTFE, maintain dimensional stability at temperatures up to 500°F, making them suitable for extreme environments.
Chemical Resistance
Chemical resistance is vital for plastics exposed to harsh environments. Materials like HDPE, polypropylene, and fluoropolymers exhibit outstanding resistance to various chemicals, acids, and solvents. This property is essential in agriculture, chemical processing, and food and beverage industries. PVDF, for example, offers excellent resistance to chemicals, UV radiation, and flames, making it versatile for diverse industrial applications. When selecting materials, factors such as chemical concentration, operating temperature range, and mechanical load must be considered to ensure optimal performance and longevity of plastic components.
Conclusion
Plastic machining has a profound influence on various industries, from medical devices to aerospace components. The selection of appropriate materials, understanding of thermal properties, and implementing specialized techniques are crucial to achieving precision and quality in machined plastic parts. To ensure dimensional stability, manufacturers must address internal stresses, control moisture, and manage temperature fluctuations. These considerations, combined with rigorous quality control measures, enable the production of high-performance plastic components that meet stringent industry requirements.
The versatility and adaptability of plastic machining open up new possibilities across diverse sectors, pushing the boundaries of what’s achievable in manufacturing. As the field continues to evolve, the importance of staying informed about material advancements and refining machining processes becomes increasingly apparent.
For your next project, contact AIP Precision Machining, where cutting-edge solutions meet quality assurance to push the boundaries of what’s possible in aerospace manufacturing. By leveraging the unique properties of plastics and employing advanced machining techniques, manufacturers can create innovative solutions that drive progress in their respective industries.
FAQs
- What is plastic machining, and how does it differ from metal machining?
Plastic machining involves precision cutting, drilling, and milling plastic materials to create custom components. Unlike metal machining, plastic machining requires specialized tools and techniques due to its lower melting points, thermal expansion, and unique mechanical properties.
- What are the most common plastics used in precision machining at AIP?
At AIP, we commonly machine high-performance plastics such as PEEK, Torlon (PAI), Ultem (PEI), and PTFE. These materials are chosen for their superior strength, thermal stability, and chemical resistance, making them ideal for demanding applications across various industries.
- How does thermal expansion affect plastic machining?
Thermal expansion in plastics can lead to dimensional changes during machining. Managing this requires precise control of machining parameters and specialized tools to maintain tight tolerances and prevent deformation, ensuring the final product meets exact specifications.
- What are the key factors to consider when selecting a plastic material for machining?
When selecting a plastic for machining, consider factors such as mechanical strength, thermal stability, chemical resistance, and the application’s specific requirements. Materials like PEEK and Torlon are preferred for high-stress environments, while PTFE is selected for its excellent temperature and chemical resistance.
- How does AIP ensure high-quality surface finishes in plastic machining?
AIP employs advanced finishing techniques to enhance the surface quality of machined plastic components. These include processes such as polishing, deburring, and coating, which ensure that the final products not only meet but exceed industry standards in terms of functionality and aesthetics.
- What industries benefit the most from plastic machining?
Plastic machining is crucial for industries that require precision and durability, including aerospace, medical devices, power and energy, and specialized industrial applications. These sectors benefit from the lightweight, strong, and chemically resistant properties of machined plastics.
- How does AIP manage quality control in plastic machining?
AIP adheres to stringent quality control processes, certified by AS 9100, ISO 13485, and ISO 9001 standards. We perform meticulous inspections at every stage of production, ensuring that all machined plastic components meet the highest standards of precision and reliability.


 The components used in aerospace applications are expected to perform over extended periods, often up to 50 years. Quality assurance is critical at every stage of the product life cycle to ensure these components can withstand continuous long-term use while maintaining safety and performance.
The components used in aerospace applications are expected to perform over extended periods, often up to 50 years. Quality assurance is critical at every stage of the product life cycle to ensure these components can withstand continuous long-term use while maintaining safety and performance. Statistical Process Control (SPC) is integral to aerospace manufacturing, enabling companies to monitor production processes in real-time. By applying SPC, manufacturers can detect deviations from a set standard, allowing for immediate corrective actions to maintain quality. This method uses statistical methods to monitor and control manufacturing processes, thereby reducing variability, enhancing product quality, and minimizing waste and costs. For instance, aerospace companies utilize SPC to analyze data collected during manufacturing to quickly identify any process that deviates from its normal operating conditions. This proactive approach helps in maintaining the rigorous standards required in aerospace parts production.
Statistical Process Control (SPC) is integral to aerospace manufacturing, enabling companies to monitor production processes in real-time. By applying SPC, manufacturers can detect deviations from a set standard, allowing for immediate corrective actions to maintain quality. This method uses statistical methods to monitor and control manufacturing processes, thereby reducing variability, enhancing product quality, and minimizing waste and costs. For instance, aerospace companies utilize SPC to analyze data collected during manufacturing to quickly identify any process that deviates from its normal operating conditions. This proactive approach helps in maintaining the rigorous standards required in aerospace parts production.
 Labeling regulations are specified under several parts of Title 21 of the Code of Federal Regulations. These regulations ensure that all medical devices are accompanied by clear, accurate labeling that informs users of the device’s intended use and any risks associated with its use. Additionally, Medical Device Reporting (MDR) is a critical postmarket surveillance tool used by the FDA to monitor device performance and track adverse events. Manufacturers, importers, and device user facilities must report any serious injuries, deaths, or safety issues encountered with the devices to the FDA.
Labeling regulations are specified under several parts of Title 21 of the Code of Federal Regulations. These regulations ensure that all medical devices are accompanied by clear, accurate labeling that informs users of the device’s intended use and any risks associated with its use. Additionally, Medical Device Reporting (MDR) is a critical postmarket surveillance tool used by the FDA to monitor device performance and track adverse events. Manufacturers, importers, and device user facilities must report any serious injuries, deaths, or safety issues encountered with the devices to the FDA. Once the quality control objectives are set, the focus shifts to the inspection and testing of medical devices. This stage is critical as it determines whether a product batch is ready for shipment. Quality control teams are responsible for conducting detailed inspections and various tests to verify that each product meets the established acceptance criteria. According to FDA’s quality system regulation, medical device manufacturers are allowed to design their own quality control tests. However, it is mandatory to maintain comprehensive documentation that substantiates the effectiveness of these tests. This documentation is crucial for meeting FDA compliance and for internal audits to assess the consistency of the quality control process.
Once the quality control objectives are set, the focus shifts to the inspection and testing of medical devices. This stage is critical as it determines whether a product batch is ready for shipment. Quality control teams are responsible for conducting detailed inspections and various tests to verify that each product meets the established acceptance criteria. According to FDA’s quality system regulation, medical device manufacturers are allowed to design their own quality control tests. However, it is mandatory to maintain comprehensive documentation that substantiates the effectiveness of these tests. This documentation is crucial for meeting FDA compliance and for internal audits to assess the consistency of the quality control process.
 Today, 3D printing is revolutionizing the aerospace industry by enabling the production of complex, lightweight components quickly and efficiently. Metal, plastic, and composite materials are used to create various parts, such as engine components, fuel nozzles, and heat exchangers.
Today, 3D printing is revolutionizing the aerospace industry by enabling the production of complex, lightweight components quickly and efficiently. Metal, plastic, and composite materials are used to create various parts, such as engine components, fuel nozzles, and heat exchangers. High-performance polymers:
High-performance polymers:
 Common Pathogens and Infection Pathways
Common Pathogens and Infection Pathways PEEK implants have demonstrated remarkable success in various clinical applications, particularly in orthopedics, dentistry, and spinal surgery. The use of PEEK materials has significantly reduced infection risks, improved implant stability, and increased patient comfort in these fields. AIP Precision Machining has been at the forefront of this innovation, providing highly precise PEEK components that meet the rigorous demands of these medical fields, thereby enhancing patient outcomes and reducing infection risks.
PEEK implants have demonstrated remarkable success in various clinical applications, particularly in orthopedics, dentistry, and spinal surgery. The use of PEEK materials has significantly reduced infection risks, improved implant stability, and increased patient comfort in these fields. AIP Precision Machining has been at the forefront of this innovation, providing highly precise PEEK components that meet the rigorous demands of these medical fields, thereby enhancing patient outcomes and reducing infection risks. Looking ahead, several potential innovations could revolutionize the use of PEEK in medical implants. One exciting prospect is the development of smart PEEK implants that incorporate sensors or drug delivery systems. These intelligent implants could monitor the healing process, detect any signs of infection, or release therapeutic agents directly at the implant site. Such advancements would enable personalized and targeted treatment, improving patient outcomes and reducing complications.
Looking ahead, several potential innovations could revolutionize the use of PEEK in medical implants. One exciting prospect is the development of smart PEEK implants that incorporate sensors or drug delivery systems. These intelligent implants could monitor the healing process, detect any signs of infection, or release therapeutic agents directly at the implant site. Such advancements would enable personalized and targeted treatment, improving patient outcomes and reducing complications.

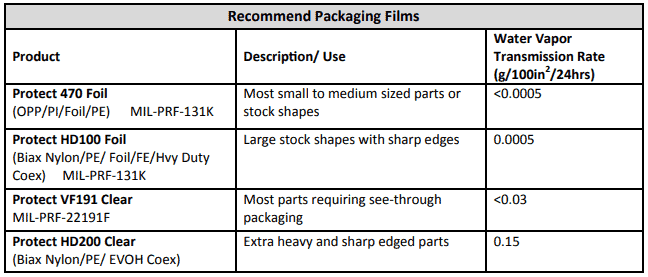
 Thermal expansion or contraction occurs when a material is exposed to temperature change and thus leads to change in all dimensions, as well as other physical properties. While this effect is most noticeable in gasses and liquids, it is also notable in solids. Softer materials such as unreinforced polymers experience greater levels of dimensional change per each degree change in temperature.
Thermal expansion or contraction occurs when a material is exposed to temperature change and thus leads to change in all dimensions, as well as other physical properties. While this effect is most noticeable in gasses and liquids, it is also notable in solids. Softer materials such as unreinforced polymers experience greater levels of dimensional change per each degree change in temperature.


 For businesses and facilities involved in the production and distribution of medical devices, FDA Registration is a requirement to machine or manufacture any plastic material that will be used in the United States medical market.
For businesses and facilities involved in the production and distribution of medical devices, FDA Registration is a requirement to machine or manufacture any plastic material that will be used in the United States medical market.