In the aerospace industry, the relentless pursuit of reliability and performance under extreme conditions is a constant. Cryogenic seals, essential components in space exploration vehicles and equipment, face the daunting task of maintaining integrity in the vastness of space.
Traditional materials often fall short in the face of extreme cryogenic temperatures and harsh environmental conditions. This article details the utilization of advanced polymers – FEP, PFA, PTFE, VESPEL®, and PCTFE – offering aerospace engineers robust solutions for their challenging cryogenic seal applications.
Challenges in Aerospace Cryogenic Sealing
Cryogenic sealing in aerospace confronts a number of challenges, primarily due to the extreme temperature fluctuations when operating in the vacuum of space. Traditional materials like elastomers, while adequate in moderate conditions, become brittle and lose elasticity at temperatures below -60°F (-51°C) or above 300°F (149°C), leading to failures in seals. This necessitates the exploration of materials that can endure such harsh conditions while maintaining their mechanical properties.
High-performance polymers not only meet but exceed the stringent requirements of aerospace applications, making them the materials of choice for cryogenic seals. Here we’ll detail the unique attributes of FEP, PFA, PTFE, VESPEL®, and PCTFE, and how each contributes to overcoming the unique challenges of space.
High-Performance Polymers: An Overview
In aerospace applications, especially for cryogenic seals, the choice of material is critical. High-performance polymers like FEP (Fluorinated Ethylene Propylene), PFA (Perfluoroalkoxy Alkane), PTFE (Polytetrafluoroethylene), VESPEL®, and PCTFE (Polychlorotrifluoroethylene) offer unique properties that make them superior to conventional sealing materials in extreme conditions.
Detailed Analysis of Each Polymer
The following list explains key properties of high-performance polymers — FEP, PFA, PTFE, VESPEL®, and PCTFE — such as chemical resistance, thermal stability, and application suitability. This comparison provides aerospace engineers with essential data for informed material selection in cryogenic environments.
FEP: This polymer is known for its exceptional resistance to aggressive chemicals, making it ideal for handling the corrosive substances often encountered in aerospace applications. FEP maintains robust physical properties even at very low temperatures, crucial for cryogenic applications. Its ability to withstand a wide temperature range (-328°F to 400°F) without losing its mechanical properties makes it an excellent choice for seals that need to maintain integrity in varying thermal conditions.
PFA: Similar to FEP in its resistance to chemicals, PFA offers even greater strength at high temperatures, up to 500°F. This thermal stability is critical in aerospace applications where seals may be exposed to rapid temperature changes. Its non-stick and low friction properties also contribute to its suitability for dynamic sealing applications where minimal resistance is desired.
PTFE: Renowned for its outstanding chemical inertness, PTFE remains stable across a wide temperature range, from -328°F to 500°F. This makes PTFE particularly capable in cryogenic sealing, where materials are often exposed to extremely low temperatures. PTFE’s low coefficient of friction minimizes wear in dynamic applications, extending the lifespan of seals under motion.
VESPEL®: This polyimide-based thermoplastic is distinguished by its exceptional heat resistance, with a continuous operating temperature up to 500°F. Its low outgassing properties at high temperatures make it suitable for vacuum applications, a common scenario in space environments. VESPEL®’s dimensional stability and creep resistance are vital in maintaining seal integrity under fluctuating pressure and temperature conditions, common in cryogenic aerospace applications.
PCTFE: Known for its low moisture absorption, PCTFE is especially useful in vacuum conditions like those found in space. Its high compressive strength and nonflammability are essential for maintaining seal integrity in the presence of cryogenic fluids. PCTFE’s excellent chemical resistance ensures durability when exposed to aggressive chemicals, a common challenge in aerospace environments.
 Each of these polymers brings a unique set of properties to the table, making them highly suitable for the demanding requirements of aerospace cryogenic seals. Their combined characteristics of chemical resistance, thermal stability, low moisture absorption, and mechanical durability under extreme conditions position them as optimal materials for aerospace applications, where failure is not an option.
Each of these polymers brings a unique set of properties to the table, making them highly suitable for the demanding requirements of aerospace cryogenic seals. Their combined characteristics of chemical resistance, thermal stability, low moisture absorption, and mechanical durability under extreme conditions position them as optimal materials for aerospace applications, where failure is not an option.
Having explored the individual properties and benefits of each high-performance polymer, it’s crucial to understand how they compare when applied to aerospace cryogenic seals.
This comparative analysis will shed light on the practical implications of choosing one polymer over another, considering the specific demands of aerospace applications. By evaluating their performance side-by-side, we can discern the most suitable material for specific cryogenic sealing applications in the aerospace industry.
Comparative Analysis: Aerospace Applications
Comparing these polymers reveals each material’s unique advantages for cryogenic seals in aerospace. PTFE’s low friction makes it suitable for dynamic seals, while VESPEL’s thermal stability is crucial for static applications in extreme temperatures. PCTFE’s moisture resistance is invaluable in the vacuum of space.
The following table compares key properties of high-performance polymers — FEP, PFA, PTFE, VESPEL®, and PCTFE — such as chemical resistance, thermal stability, and application suitability. This comparison provides aerospace engineers with essential data for informed material selection in cryogenic environments.

| Polymer | Chemical Resistance | Thermal Stability | Low Friction | Outgassing at High Temp | Moisture Absorption | Applications in Aerospace |
| FEP | High | -328°F to 400°F | Yes | Low | Low | Dynamic Seals |
| PFA | High | -328°F to 500°F | Yes | Low | Low | Dynamic Seals |
| PTFE | Highest | -328°F to 500°F | Yes | Low | Low | Dynamic Seals |
| VESPEL® | High | Up to 500°F | No | Minimal | Small amount | Static Applications |
| PCTFE | High | Low | No | N/A | Low | Static Applications |
The comparative analysis underscores the tailored suitability of each polymer for specific aerospace applications. However, selecting the right material is only part of the equation. The next critical step is the precision machining of these materials into functional cryogenic seals. This section will discuss the machining considerations essential for realizing the potential of these high-performance polymers in aerospace applications. AIP Precision Machining’s expertise in this domain ensures that the advanced properties of these polymers are fully harnessed in the final cryogenic seal products.
Machining Considerations for Aerospace Cryogenic Seals
The machining of materials for cryogenic seals demands meticulous attention to detail. Given the extreme conditions of space, even minuscule discrepancies in the dimensions of a seal can lead to catastrophic outcomes. It is here that the micro-precision in machining comes into play, especially for high-performance polymers like FEP, PFA, PTFE, VESPEL®, and PCTFE.
The precision required for these polymers is of the highest order, often necessitating tolerances as tight as 0.002 mm. This level of accuracy is imperative to ensure that each seal perfectly fits its designated space, providing an airtight barrier against the harsh conditions of outer space.
Any deviation, however minor, can compromise the integrity of the seal, leading to potential failure of the system it is meant to protect. In the vacuum of space, where repair is not a viable option, the reliability of every component is crucial.
For polymers like PTFE and VESPEL®, known for their low friction and thermal stability, the precision in machining also dictates their performance over time. Inaccuracies in dimensions can lead to increased wear and tear, reducing the longevity of these components in critical aerospace applications.
Similarly, for PCTFE and FEP, known for their chemical resistance and low moisture absorption, precision machining ensures that their properties are fully utilized, maintaining the seal’s integrity in the presence of volatile cryogenic fluids.
AIP Precision Machining has routinely demonstrated the capability to achieve extreme precision of up to 0.002 mm. AIP stands at the forefront of machining high-performance polymers for aerospace applications, as their experienced technicians and state-of-the-art technology ensure that each component is machined to exact specifications, leaving no room for error.
By entrusting the machining of cryogenic seals to AIP, aerospace engineers can significantly mitigate the risks associated with material and manufacturing inaccuracies, ensuring the reliability and success of their space missions.
In conclusion, the precision in machining cryogenic seals from high-performance polymers is not just a matter of technical requirement but a critical factor in the success of aerospace projects. The utilization of advanced machining capabilities, such as those offered by AIP Precision Machining, is crucial in ensuring that these components meet the stringent demands of space, where every micron counts and any error can lead to irrevocable consequences.
———————
Citations:
- https://pubs.aip.org/aip/acp/article/985/1/422/990643/DEVELOPMENT-OF-A-CRYOGENIC-GATE-VALVE-WITH-ROBUST
- https://www.sciencedirect.com/science/article/abs/pii/S0042207X76800103?via%3Dihub


 2.3 Market Opportunities
2.3 Market Opportunities 4. Regional Analysis
4. Regional Analysis In a case study for
In a case study for  In the medical world, devices often come into contact with a variety of chemicals, be it medications, sterilization agents, or bodily fluids. PEEK is chemically inert, resisting potential degradation or reactions that could compromise the device’s functionality or patient safety. Furthermore, PEEK devices can be sterilized using standard medical sterilization techniques without compromising the material’s integrity.
In the medical world, devices often come into contact with a variety of chemicals, be it medications, sterilization agents, or bodily fluids. PEEK is chemically inert, resisting potential degradation or reactions that could compromise the device’s functionality or patient safety. Furthermore, PEEK devices can be sterilized using standard medical sterilization techniques without compromising the material’s integrity.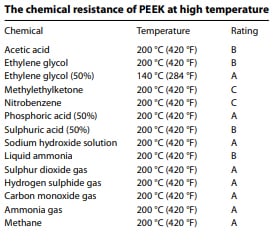 The chart at the right shows the chemical resistance of PEEK at 200°C (420°F). PEEK exhibits excellent resistance to a wide range of organic and inorganic chemicals. The compatibility of PEEK with many chemicals at 20 °C (68 °F) has been investigated and the results for unreinforced grades are favorable. PEEK is compatible with almost any of the solvents used in HPLC. The only solvent which will attack PEEK are concentrated nitric acid and sulfuric acids.
The chart at the right shows the chemical resistance of PEEK at 200°C (420°F). PEEK exhibits excellent resistance to a wide range of organic and inorganic chemicals. The compatibility of PEEK with many chemicals at 20 °C (68 °F) has been investigated and the results for unreinforced grades are favorable. PEEK is compatible with almost any of the solvents used in HPLC. The only solvent which will attack PEEK are concentrated nitric acid and sulfuric acids.
 One of the primary growth drivers for the aerospace parts manufacturing industry is the increasing demand for aircraft maintenance and replacement. As commercial airlines, military aircraft, and general aviation operations continue to expand, the need for high-quality, reliable aircraft components escalates. Furthermore, with aircraft often exposed to harsh environmental conditions, the need for regular maintenance and part replacement is paramount, ensuring operational safety and efficiency.
One of the primary growth drivers for the aerospace parts manufacturing industry is the increasing demand for aircraft maintenance and replacement. As commercial airlines, military aircraft, and general aviation operations continue to expand, the need for high-quality, reliable aircraft components escalates. Furthermore, with aircraft often exposed to harsh environmental conditions, the need for regular maintenance and part replacement is paramount, ensuring operational safety and efficiency.
 Predictive maintenance, powered by big data and analytics, is transforming the aviation industry. By leveraging flight-recorded data, airlines can significantly reduce maintenance expenditures and enhance operational efficiency. This trend is creating unique growth opportunities for aerospace parts manufacturers, enabling them to meet evolving industry requirements.
Predictive maintenance, powered by big data and analytics, is transforming the aviation industry. By leveraging flight-recorded data, airlines can significantly reduce maintenance expenditures and enhance operational efficiency. This trend is creating unique growth opportunities for aerospace parts manufacturers, enabling them to meet evolving industry requirements.
 The temperature adaptability of these plastics is demonstrated by their operational reliability within the space environment, where temperatures range from -150°C to 130°C, and they exhibit resistance to elevated temperatures in rocket engine applications. Exhibiting optimal flammability characteristics, these materials are compatible with both liquid oxygen (LOX) and gaseous oxygen (GOX), critical for maintaining safety in the highly reactive environments of spacecraft.
The temperature adaptability of these plastics is demonstrated by their operational reliability within the space environment, where temperatures range from -150°C to 130°C, and they exhibit resistance to elevated temperatures in rocket engine applications. Exhibiting optimal flammability characteristics, these materials are compatible with both liquid oxygen (LOX) and gaseous oxygen (GOX), critical for maintaining safety in the highly reactive environments of spacecraft.
 Ultem’s notable characteristics are high processability, dimensional stability, environmental stress resistance, and flammability resistance, all of which are critical for the harsh and unpredictable conditions of space. It also offers long-term heat resistance, which is a significant factor for components exposed to extreme temperatures, which is beneficial for rocket launches.
Ultem’s notable characteristics are high processability, dimensional stability, environmental stress resistance, and flammability resistance, all of which are critical for the harsh and unpredictable conditions of space. It also offers long-term heat resistance, which is a significant factor for components exposed to extreme temperatures, which is beneficial for rocket launches. Torlon also offers excellent wear and radiation resistance, both of which are essential properties for materials used in space environments. Inherent low flammability and smoke emission make it an ideal material for high temperature and potentially hazardous conditions present in rocket propulsion systems.
Torlon also offers excellent wear and radiation resistance, both of which are essential properties for materials used in space environments. Inherent low flammability and smoke emission make it an ideal material for high temperature and potentially hazardous conditions present in rocket propulsion systems. One of the remarkable characteristics of Vespel is its high-temperature resistance. This makes it ideal for use in the space industry where materials are frequently subjected to extreme temperatures. Furthermore, Vespel does not exhibit significant outgassing, even at high temperatures. This makes it useful for manufacturing lightweight heat shields and crucible support structures for spacecraft and rocket propulsion systems, where any outgassing could cause contamination and performance issues.
One of the remarkable characteristics of Vespel is its high-temperature resistance. This makes it ideal for use in the space industry where materials are frequently subjected to extreme temperatures. Furthermore, Vespel does not exhibit significant outgassing, even at high temperatures. This makes it useful for manufacturing lightweight heat shields and crucible support structures for spacecraft and rocket propulsion systems, where any outgassing could cause contamination and performance issues.
 Since then, PEEK has only expanded as a performance biomaterial for instrumented spine surgery. In the United States, spine fusion is one of the leading surgeries for patients who suffer from chronic neck and back pain that does not respond to preliminary treatments.
Since then, PEEK has only expanded as a performance biomaterial for instrumented spine surgery. In the United States, spine fusion is one of the leading surgeries for patients who suffer from chronic neck and back pain that does not respond to preliminary treatments. The fields of orthopedics and spinal fusion continue to research new methods for best practices in the industry. Over the last three decades, medical-grade PEEK has established itself as the performance biomaterial of choice for surgeons and OEMs. Medical device design demands the highest level of sanitation, biocompatibility, and precision in one of the most extreme environments, the human body.
The fields of orthopedics and spinal fusion continue to research new methods for best practices in the industry. Over the last three decades, medical-grade PEEK has established itself as the performance biomaterial of choice for surgeons and OEMs. Medical device design demands the highest level of sanitation, biocompatibility, and precision in one of the most extreme environments, the human body.
 Besides incredible thermal stability and resistance rivaling aluminum, copper, and steel, Torlon® is well known for its strength under pressure and chemical resistance. Torlon’s benefits include the following:
Besides incredible thermal stability and resistance rivaling aluminum, copper, and steel, Torlon® is well known for its strength under pressure and chemical resistance. Torlon’s benefits include the following: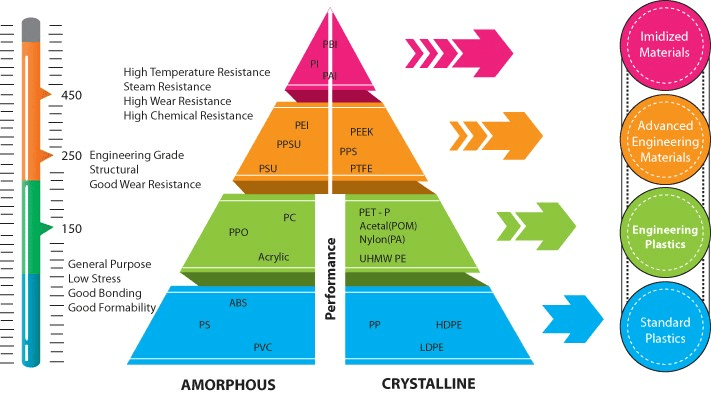


 TECATEC PEEK MT CW50® black plates are based on Victrex® PEEK that is reinforced with 50% vol. carbon fibers. This carbon fiber reinforcement elevates the stiffness and strength to be many times those of plates made from unreinforced PEEK or plates with short fiber reinforcement.
TECATEC PEEK MT CW50® black plates are based on Victrex® PEEK that is reinforced with 50% vol. carbon fibers. This carbon fiber reinforcement elevates the stiffness and strength to be many times those of plates made from unreinforced PEEK or plates with short fiber reinforcement.
 VESPEL
VESPEL KEL-F®
KEL-F® PTFE
PTFE