
The aerospace industry now accounts for nearly 16% of the total revenue generated by the additive manufacturing industry. Precision 3D printers have achieved remarkable milestones, exemplified by Relativity Space launching the world’s first 3D-printed rocket in March 2023. Despite these advancements, 3D printing technology still faces significant challenges, including high costs, low printing speeds, limited part sizes, and strength issues.
While different 3D printing technologies offer varying capabilities regarding tolerances—such as MJF at ±0.3% (±0.2 mm), SLS at ±0.3% (±0.3 mm), SLA at ±0.5% (±0.2 mm), and FDM at ±0.5% (±0.5 mm)—they all struggle with fundamental limitations. This variance becomes particularly problematic in highly regulated industries like aerospace and medical device manufacturing, where even slight dimensional inaccuracies can have serious consequences.
Although processes like SLA provide fine finishes and high accuracy, materials undergo shrinkage during curing, creating internal stresses that affect dimensional accuracy. This article examines why even the most advanced 3D printing systems fall short in meeting the stringent requirements of regulated manufacturing environments, and how hybrid manufacturing approaches combining additive and subtractive methods offer a more viable solution.
Dimensional Tolerance Challenges in Precision 3D Printing
Dimensional accuracy remains a significant challenge for manufacturers relying on additive technologies, even with the most sophisticated equipment available. Resolution specifications alone fail to guarantee accurate parts, as multiple factors affect the final dimensional accuracy of printed components.
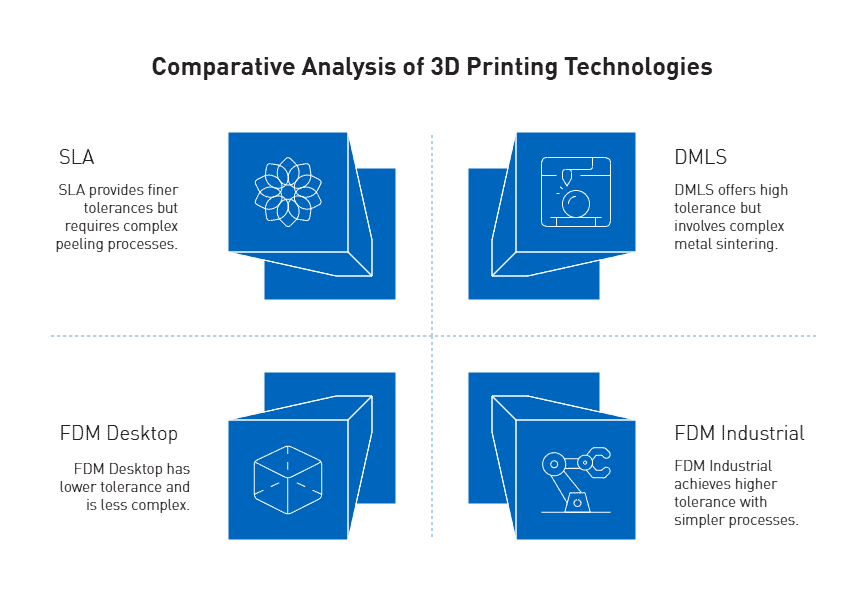
Inconsistent tolerances in FDM vs SLA vs DMLS
Each 3D printing technology offers notably different tolerance capabilities. FDM systems typically achieve tolerances of ±0.5% (lower limit: ±0.5 mm) for desktop printers and ±0.15% (lower limit: ±0.2 mm) for industrial machines. Furthermore, FDM parts frequently suffer from warping and shrinkage as layers cool at different rates, creating internal stresses that deform prints.
In contrast, SLA technology produces finer tolerances of approximately ±0.2% (lower limit: ±0.1 mm). However, the peeling process during printing can introduce dimensional inaccuracies as pulling forces cause soft prints to bend slightly.
DMLS offers tolerances of ±0.2% (lower limit: ±0.1-0.2 mm), yet the high heat involved in metal sintering often leads to shrinkage issues that must be compensated for in design.
3D printing tolerance for moving parts and assemblies
Achieving functional assemblies with precision 3D printers requires specific clearance considerations. For proper fit between components, manufacturers typically apply clearance gaps of 0.005″ (0.127 mm) for tight fits, 0.010″ (0.254 mm) for normal fits, and 0.020″ (0.508 mm) for loose fits.
Additionally, holes in 3D printed parts consistently print undersized due to limitations of the STL file format, which builds circles from straight-edged triangles. This characteristic necessitates accounting for these deviations when designing critical features.
Impact of anisotropic properties on part accuracy
Layer-by-layer fabrication inherently creates anisotropic characteristics in printed components. Mechanical testing reveals that build orientation significantly influences final properties, with parts built in upright orientation demonstrating especially poor performance.
Research confirms that raster direction substantially affects fracture behavior in FDM parts. For instance, SLA parts printed in 90° orientation exhibit greater strength but increased brittleness compared to other orientations because previously cured layers receive additional laser exposure.
This orientation-dependent performance becomes particularly problematic in regulated industries where consistent mechanical properties are essential across all dimensions. Consequently, the anisotropic nature of 3D printed parts often necessitates subsequent machining operations to achieve the uniform properties and tight tolerances required for aerospace and medical applications.
Material and Surface Limitations in Regulated Applications
Surface finish stands as a fundamental barrier to widespread adoption of additive manufacturing in regulated industries. Surface roughness, a critical quality indicator, directly impacts mechanical, physical, and tribological properties of printed components.
Surface roughness and porosity in aerospace-grade polymers
Internal porosity poses a significant challenge in 3D printed parts, as these invisible voids reduce component density and potentially lead to cracks, leaks, and fatigue. Recent innovations at Oak Ridge National Laboratory have demonstrated that vacuum-assisted extrusion can reduce internal porosity by up to 75% in large-scale polymer prints. This advancement is crucial given that surface irregularities disrupt boundary layer flow and accelerate the transition from laminar to turbulent flow, particularly in aerospace applications such as UAV propellers.
Moreover, surface roughness values vary dramatically between materials and printing technologies. For instance, PLA exhibits higher arithmetic mean roughness values (16.2-17.6 μm) compared to PETG (9.0-10.8 μm) in standard quality prints. These variations significantly influence functional performance, with rougher surfaces leading to enhanced aerodynamic drag and irregular airflow patterns.
Limitations of PEEK additive manufacturing for FDA-compliant parts
PEEK, despite its popularity in medical applications, presents substantial manufacturing challenges. Its high melting temperature, semi-crystalline structure, and significant cooling shrinkage rate increase process complexity and result in uncontrollable properties of 3D-printed parts. Indeed, the crystallization properties of PEEK are greatly affected by thermal history, requiring precise control of printing temperature, ambient temperature, and heat treatment conditions.
Another major obstacle lies in PEEK’s biological inertness, which hinders integration with bone and soft tissue—a critical requirement for medical implants. For dental applications specifically, printing high-resolution small structures (<15 mm) with fine threads proves extremely challenging, primarily because extruding PEEK through small diameter nozzles (<0.2 mm) is difficult due to the polymer’s high melt viscosity.
Post-processing requirements for biocompatible components
Biocompatibility certifications necessitate rigorous post-processing protocols. For instance, to achieve compliance with ISO 10993-5:2009 (cytotoxicity), ISO 10993-10:2021 (sensitization), and ISO 10993-23:2021 (irritation) standards, parts must undergo specific washing and curing processes. Typically, this involves multiple wash cycles in IPA or ethanol, followed by air drying and thermal curing.
File transfer also poses regulatory challenges, as the FDA expresses concerns about consistency across file formats. The ability to verify initial design intent throughout the manufacturing process is essential for traceability and audit purposes. Consequently, post-processing becomes a critical consideration for manufacturers seeking to produce FDA-compliant components.
Why Precision Alone Isn’t Enough for Regulated Manufacturing
Regulated manufacturing environments demand more than just dimensional accuracy from production methods. Precise tolerances serve as merely one aspect of a comprehensive quality system that must satisfy rigorous industry standards.
AS9100 and ISO 13485 compliance requirements
Beyond precise dimensions, AS9100 (aerospace) and ISO 13485 (medical devices) certifications require exhaustive documentation of all manufacturing processes. These standards necessitate controlled procedures, risk management protocols, and regular internal audits. For instance, ISO 13485 mandates complete product identification throughout manufacturing, thorough validation of all processes, and strict control of nonconforming products. Similarly, AS9100 requires detailed first article inspection reports documenting 100% of product characteristics.
Traceability and repeatability in medical device manufacturing
Medical device manufacturing mandates unbroken traceability from raw material to finished product. Every component must be traceable to its manufacturing batch, machine parameters, and operator. Nevertheless, 3D printing creates challenges in this domain as material properties can vary substantially between print jobs due to environmental factors like ambient humidity or temperature fluctuations. These variations undermine the consistency required for validated medical manufacturing processes.
Limitations of 3D printing in achieving validated processes
Process validation, a cornerstone requirement in regulated manufacturing, demands that manufacturers prove their processes consistently produce the same results. Unfortunately, additive manufacturing processes face inherent difficulties achieving this standard. Factors including thermal history, layer adhesion variability, and support structure removal introduce unpredictability that complicates validation efforts. Therefore, while precision 3D printers excel at creating complex geometries, they rarely satisfy the stringent validation requirements of regulated environments without substantial post-processing and secondary operations.
Hybrid Manufacturing: Bridging the Gap with CNC Machining
Hybrid manufacturing is a practical solution that combines the complex geometry capabilities of additive manufacturing with the superior surface finish and tight tolerances of precision machining. This integrated approach effectively addresses the fundamental limitations of standalone 3D printing systems.
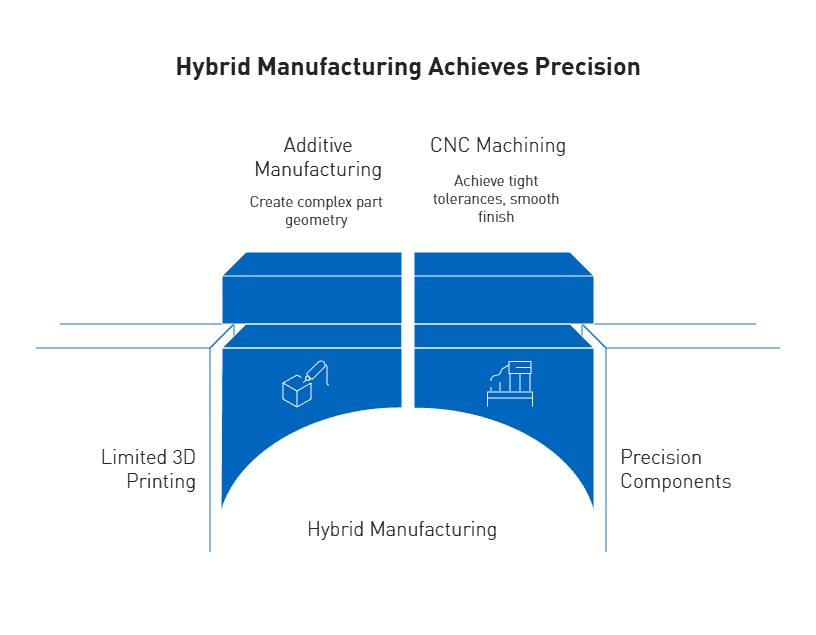
Subtractive finishing of 3D printed parts for tight tolerances
Hybrid processes enhance dimensional accuracy dramatically—from Ra30μm in selective laser melting to Ra0.4μm after CNC finishing. Ultimately, this approach enables components to meet the micron-level tolerances required for critical applications. While precision 3D printers typically achieve tolerances around 0.1 mm, CNC machining elevates this to 0.025 mm, making hybrid manufacturing ideal for moving parts and assemblies.
Machined PEEK components for aerospace and medical use
PEEK stands out as an exceptional polymer for regulated applications, offering remarkable properties:
- Maintains flexural and tensile properties at temperatures exceeding 482°F
- Withstands over 1,500 steam autoclave cycles without significant degradation
- Demonstrates exceptional resistance to wear, heat, and radiation
These characteristics make machined PEEK components essential for critical aerospace and medical applications where thermal, chemical, and combustion properties are paramount.
AIP’s polymer-only expertise and certified hybrid workflows
Operating under ISO 13485:2016 compliance with FDA registration status, AIP has achieved tolerance capabilities reaching ±0.002 mm. By exclusively machining plastics, they eliminate metallic cross-contamination risk—vital for biocompatible applications where material purity directly impacts patient safety.
Ready to bridge the gap between additive innovation and certified performance? Partner with AIP Precision Machining to transform your precision 3D printed parts into mission-critical components that meet the highest regulatory and engineering standards. Contact AIP’s engineering team today to discuss hybrid manufacturing solutions built for compliance, consistency, and performance.
Conclusion
Precision 3D printers have advanced rapidly, offering new possibilities in design complexity and rapid prototyping. However, when it comes to regulated manufacturing environments—where compliance, repeatability, and micron-level tolerances are non-negotiable—additive alone isn’t enough.
Across all major 3D printing technologies, issues like dimensional variability, anisotropic mechanical properties, and rough surface finishes continue to challenge part consistency. Materials such as PEEK, ideal for aerospace and medical applications, become difficult to control during additive processing due to shrinkage, crystallization, and thermal instability.
More critically, certifications like AS9100 and ISO 13485 require validated processes, traceability, and consistent outcomes—criteria that additive methods often fail to meet without secondary operations.
This is where hybrid manufacturing provides a decisive advantage. By combining the design freedom of additive with the precision, surface finish, and process control of CNC machining, manufacturers can meet both engineering and regulatory demands.
At AIP Precision Machining, we specialize in this integrated approach. With over 40 years of experience in polymer-only machining, and certified processes for high-performance plastics, we help clients turn printed parts into production-ready, regulation-compliant components.






