Biocompatible plastics have revolutionized the medical implant industry, offering distinctive advantages over traditional materials for long-term implantation. These specialized synthetic polymers provide superior performance when used within the human body, ensuring devices don’t trigger adverse reactions. Specifically, PEEK (polyether ether ketone) stands as one of the most common polymer choices for implantable medical devices, available in FDA and USP Class VI approved medical grades.
Since receiving FDA approval in 1998, PEEK has been recognized as a viable polymer for bone substitution. What makes this high-performance thermoplastic particularly valuable is its mechanical properties, which closely resemble those of human bone, providing necessary support and stability in structural implants. Furthermore, PEEK exhibits excellent biocompatibility for long-term implantation while demonstrating remarkable chemical resistance. Although it can be an expensive option, the combination of properties and reliability makes PEEK well worth the investment for medical applications. This guide examines the selection criteria for implant-grade biocompatible plastics, from initial material testing through FDA approval processes, helping engineers and regulatory professionals navigate the complex landscape of medical-grade polymers.
Understanding the Role of Biocompatible Plastics in Implants
Medical device engineers increasingly select polymers over traditional metallic materials for implantable applications. This shift stems from several critical advantages that biocompatible plastics offer in addressing the complex challenges of long-term implantation.
Why Polymers Are Replacing Metals in Medical Devices
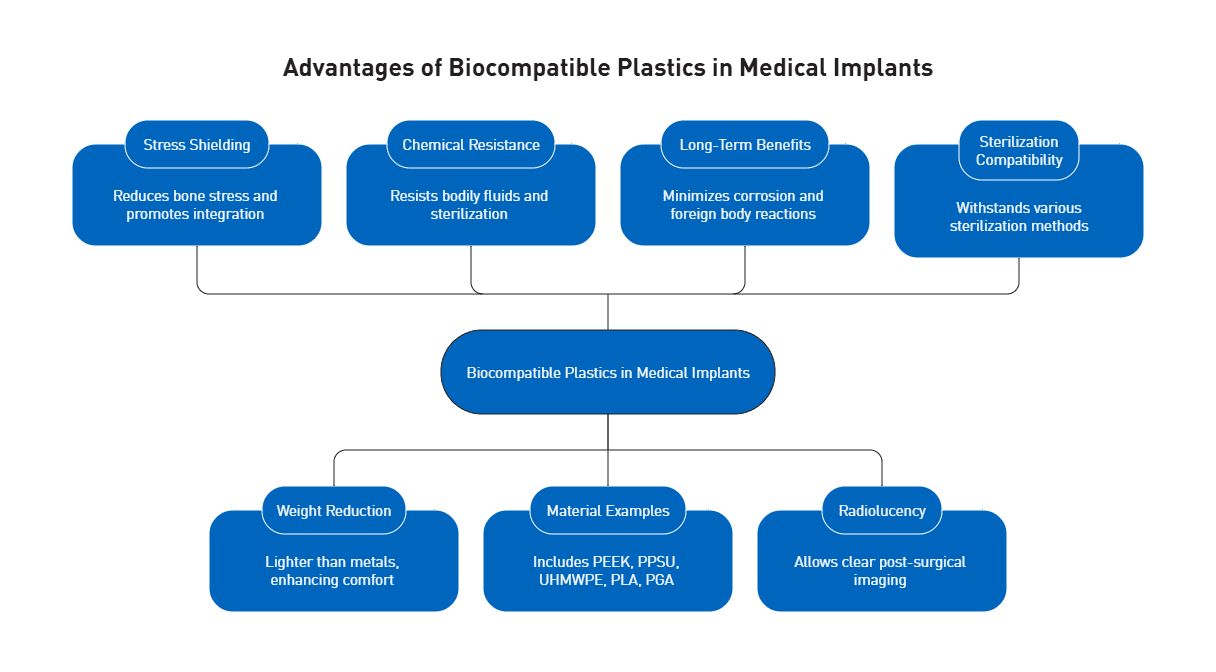
The phenomenon of “stress shielding” represents a significant issue with metallic implants. When stainless steel components are implanted, the bone experiences reduced mechanical stimulation due to the implant’s rigidity, eventually leading to loosening over time. In contrast, polymers like PEEK demonstrate flexibility closely matching natural bone structure. This mechanical compatibility reduces interface stress between the implant and surrounding tissue, promoting better long-term integration. Additionally, biocompatible plastics weigh substantially less than metals—up to ten times lighter—while offering superior chemical and corrosion resistance against bodily fluids and sterilization processes.
Examples of Implantable Device Materials in Use Today
The medical implant landscape features several prominent biocompatible polymers. The four traditional mainstay polymers—polyvinyl chloride (PVC), polypropylene (PP), polyethylene (PE), and polystyrene (PS)—continue to serve various applications. However, more specialized materials have emerged for implantable use. PEEK dominates in spinal fusion cages, having largely replaced titanium due to its bone-like mechanical properties. PPSU (polyphenylsulfone) offers exceptional strength, dimensional stability, and compatibility with bodily fluids. Ultra-high molecular weight polyethylene (UHMWPE) serves as a critical component in nearly all total knee replacements and many hip replacements, providing necessary cushioning and movement. Moreover, bioresorbable options like polylactic acid (PLA) and polyglycolic acid (PGA) offer temporary structural support that gradually dissolves as the body heals.
Benefits of Biocompatible Plastics for Long-Term Use
Biocompatible plastics deliver numerous advantages for extended implantation periods. Their inherent resistance to corrosion eliminates degradation concerns that plague metal implants. Furthermore, these materials minimize foreign body reactions—a critical factor in implant longevity. The radiolucency of polymers like PEEK facilitates post-surgical imaging without interference, allowing for more accurate monitoring of healing and potential complications. Notably, the flexibility to modify surface properties through various treatments enhances osseointegration capabilities. Biocompatible polymers also withstand multiple sterilization methods including steam, ethylene oxide, and gamma radiation without compromising structural integrity.
Mechanical and Biological Properties of Common Polymers
The mechanical and biological characteristics of polymers determine their suitability for specific implant applications and their long-term performance within the human body.
Elastic Modulus and Bone-Matching Behavior
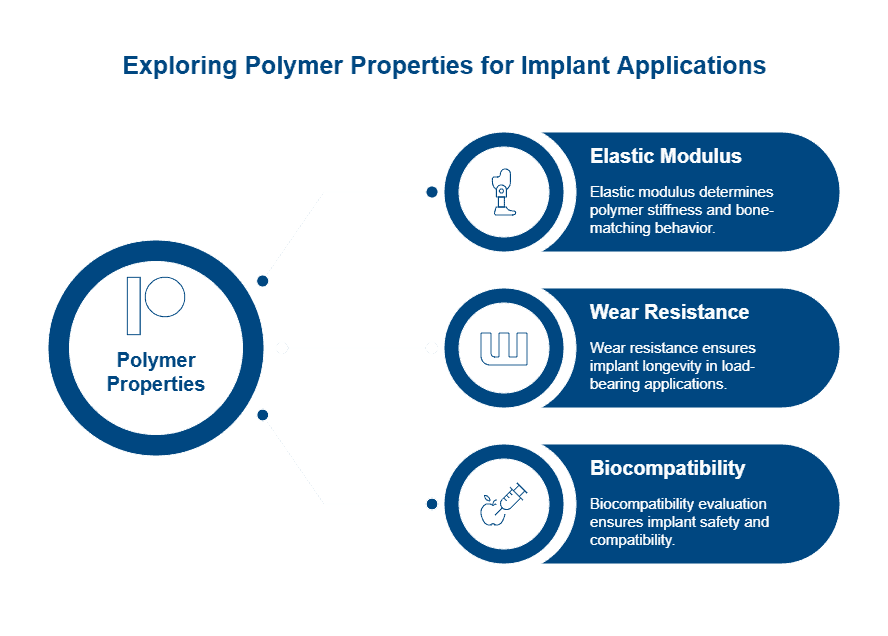
The elastic modulus (Young’s modulus) of polymers represents their stiffness or resistance to deformation. This property is crucial for implant selection as bone’s natural modulus ranges from 4 to 30 GPa. Biomaterial engineers seek polymers with comparable values to prevent stress shielding—a phenomenon where overly rigid implants cause bone resorption. Unlike metals with moduli approximately 10 times higher than bone, titanium and its alloys demonstrate values roughly half that of stainless steel, reducing stress shielding risk.
Various factors influence polymer elasticity, including molecular structure, chain flexibility, cross-linking degree, and environmental conditions. Testing methods include ultrasonic pulse velocity measurement, flexural resistance evaluation, and microhardness indentation—with the latter proving effective for estimating elastic moduli of filled polymers.
Wear Resistance and Fatigue Life in Load-Bearing Applications
Implant longevity primarily depends on wear resistance and fatigue life, especially in joint replacements. Primary failure modes include wear, yielding, creep, fatigue, fracture, and delamination. Wear-induced biological failure commonly occurs around hip replacements when debris accumulates, triggering inflammation, bone loss, and aseptic loosening.
Highly crosslinked ultrahigh molecular weight polyethylene (HXLPE) demonstrates superior wear resistance compared to non-crosslinked UHMWPE, consequently reducing particulate debris risk—albeit with diminished toughness and fatigue resistance. Fatigue testing shows biodegraded specimens exhibit 81-95% lower fatigue lifetime compared to non-degraded samples at stress levels between 2.5-10 MPa.
Biocompatibility and Cytotoxicity Profiles
Biocompatibility evaluation follows ISO 10993 standards within a risk management framework. This process begins with assessing material components, manufacturing processes, anatomical location, and exposure duration. The cytotoxicity test, described in ISO 10993-5, serves as the most sensitive initial biocompatibility screening.
Testing typically involves extracting devices in cell culture media and exposing mouse fibroblast (L929) cells to evaluate toxicity. Evaluation methods include qualitative microscopic assessment (grading 0-4) or quantitative measurement using tetrazolium dye to assess cell metabolic activity. Importantly, a failed cytotoxicity test doesn’t necessarily indicate clinical safety risk but requires systematic investigation.
Manufacturing and Post-Processing Considerations
The precision manufacturing of biocompatible plastics presents unique challenges that directly impact implant performance. Properly executed production processes ensure device efficacy while inappropriate methods can compromise biocompatibility and mechanical integrity.
Plastic Machining for Medical Devices: Tolerances and Challenges
Manufacturing biocompatible implants requires exceptional precision. CNC machining can achieve tolerances as tight as ±0.001″ for critical medical components, nevertheless material-specific factors affect achievable precision. Acetal and PEEK components maintain tolerances of +/-.001″, whereas nylon requires more generous allowances at +/-.002″. Furthermore, thermal management presents a significant challenge as polymers have higher coefficients of thermal expansion than metals. Preventing stress-induced warping requires specialized techniques—initially performing rough machining followed by a relaxation period allows internal stresses to dissipate before finishing operations.
Injection Molding vs. Additive Manufacturing for PEEK
Injection molding remains the gold standard for high-volume production of biocompatible implants, offering exceptional dimensional accuracy within tolerances as tight as +/- 0.005 inches. In contrast, additive manufacturing (AM) provides design freedom for complex geometries without sophisticated tooling. Despite these advantages, 3D-printed parts typically demonstrate inferior mechanical properties—approximately 80% lower Young’s modulus and 70% reduced tensile strength compared to injection-molded equivalents. Interestingly, research shows adding 1 wt% IF-WS2 nanoparticles to 3D printed PEEK can overcome reduced stiffness, resulting in properties surpassing unfilled injection-molded PEEK.
Surface Modification for Improved Osseointegration
Surface modification significantly enhances implant integration with bone tissue. Techniques including sand-blasting, large-grit, and acid-etching (SLA) improve mechanical interlocking with adjacent bone. For optimal results, biomimetic approaches create surfaces that mimic natural bone’s micro-nano-scale hierarchical structures. Additionally, coating implants with hydroxyapatite or other calcium phosphates promotes faster osseointegration through improved bioactivity. Surface treatments that incorporate zinc ions demonstrate enhanced antimicrobial properties while stimulating osteoblasts through interaction with Runx2 transcription factors.
Sterilization Effects on Polymer Integrity
Sterilization methods significantly impact polymer performance. High-energy methods (gamma and E-beam irradiation) induce polymer chain scission and crosslinking, altering molecular weight, crystallinity, and mechanical properties. These effects are more pronounced in degradable polymers with polar ester linkages. Steam sterilization (121°C/15min or 134°C/3min) affects surface roughness parameters—reducing maximum height (Sz value) from 41.30μm to 27.49μm in PEEK samples. Consequently, manufacturers must evaluate post-sterilization properties to ensure clinical functionality.
Ready to prototype your next implantable component? Request a quote from AIP’s medical machining team.
Compliance and Approval for Medical-Grade Polymers
Regulatory approval frameworks ensure biocompatible polymers meet stringent safety standards before clinical use. These systems protect patients through comprehensive material evaluation protocols.
ISO 10993 Plastics Testing: Cytotoxicity to Implantation
ISO 10993 serves as the primary standard for biological evaluation of medical devices within a risk management process. The standard requires assessment of physical and chemical characteristics plus exposure parameters including nature, degree, frequency, and duration of bodily contact. Testing typically begins with cytotoxicity (ISO 10993-5), the most sensitive initial biocompatibility screening. For polymers, ISO 10993-13 provides specific guidance on identifying degradation products through accelerated and real-time testing methods.
FDA-Approved Polymers and Device Class Mapping
The FDA categorizes implantable devices primarily as Class II or Class III based on risk factors. Classification considerations include treatment conditions, implantation location, device characteristics, and implantation duration. Class II devices can proceed through 510(k) clearance if risks are identifiable and manageable, whereas Class III devices typically require clinical data through the more rigorous Premarket Approval (PMA) pathway.
Implant Grade Biocompatible Plastics: Certification Process
Implant-grade materials undergo stringent certification requirements to verify biocompatibility, stability, and durability. Documentation must demonstrate non-toxicity, non-carcinogenicity, and non-irritation to biological tissues. USP Class VI represents one certification option, requiring systemic injection, intracutaneous, and implantation tests. Nevertheless, many regulatory authorities consider ISO 10993 more comprehensive, as USP Class VI lacks risk assessment components.
Navigating Custom Device Exemptions and PMA Pathways
The PMA review involves four steps: administrative review, scientific evaluation, advisory committee assessment, and final decision. Alternatively, custom device exemptions exist for unique patient needs, provided devices meet specific criteria including production limits of five units yearly per device type. Such devices must treat sufficiently rare conditions where clinical trials would be impractical.
Conclusion
The selection of biocompatible plastics for implantable medical devices demands careful evaluation of mechanical performance, biological compatibility, and regulatory compliance. Advanced polymers like PEEK, PPSU, and UHMWPE offer advantages over metals through bone-matching elasticity, resistance to stress shielding, and superior chemical durability.
Precision manufacturing—whether through CNC machining or injection molding—directly impacts implant integrity. Surface treatments and sterilization methods must be tailored to each material to maintain structural and biological performance. At the regulatory level, ISO 10993 testing and proper FDA device classification are essential for approval.
For medical engineers and OEMs, choosing the right polymer translates to safer, more effective devices. As polymer technology advances, the focus will remain on enhancing osseointegration, longevity, and patient outcomes.
Ready to prototype your next implantable component? Request a quote from AIP’s medical machining team.
FAQs
Q1. What are the main advantages of using biocompatible plastics in medical implants?
Biocompatible plastics offer several advantages over traditional metals, including reduced stress shielding, lighter weight, superior chemical resistance, and better compatibility with the human body. They also allow for improved imaging and can be modified for enhanced osseointegration.
Q2. How does PEEK compare to other materials for implantable medical devices?
PEEK (polyether ether ketone) is a popular choice for implantable devices due to its mechanical properties that closely resemble human bone. It offers excellent biocompatibility, chemical resistance, and has FDA approval for long-term implantation. While more expensive than some alternatives, its combination of properties makes it valuable for many medical applications.
Q3. What are the key considerations in manufacturing biocompatible plastic implants?
Manufacturing considerations include achieving tight tolerances through CNC machining or injection molding, managing thermal expansion during production, and applying appropriate surface modifications for improved osseointegration. Additionally, the effects of sterilization methods on polymer integrity must be carefully evaluated.
Q4. How are biocompatible plastics tested for safety and regulatory approval?
Biocompatible plastics undergo rigorous testing according to ISO 10993 standards, which include cytotoxicity, sensitization, and implantation tests. The FDA classifies implantable devices based on risk factors, with most falling under Class II or III. Approval processes may involve 510(k) clearance or the more comprehensive Premarket Approval (PMA) pathway.
Q5. What future developments can we expect in biocompatible plastics for medical implants?
Future advancements in biocompatible plastics are likely to focus on improving osseointegration capabilities, enhancing wear resistance, and developing innovative surface treatments. Research may also aim to further refine the mechanical properties of polymers to better match those of human tissues and improve long-term implant performance.