A discussion with the plastics pros at AIP on how to choose the right plastic for your medical application
When it comes to selecting a polymer for a medical application, there are a myriad of factors that play into deciding which grade of polymer is the best candidate. Of course, the number one concern in medical device design is safety of human life.
For this reason, trends in medical device design are moving toward miniaturization and portability. Additionally, sterilization and cleanliness continue to lead design considerations globally, requiring devices to withstand a range of chemicals and sterilization techniques.
As a precision plastics machining company, AIP has over 35+ years of experience working with medical OEMs to develop parts for critical medical devices. In this issue of our monthly blog, we will discuss what makes a polymer medical grade and how to choose the right polymer for a medical device application.
What are medical grade plastics?
Let’s begin with what a medical grade plastic is. Medical-grade plastics refer to plastics used to make medical products, products for in vitro diagnostics and primary packaging for pharmaceuticals.
Most importantly, plastics used in the medical field are coming in contact with human tissue, fluids, chemicals, drugs and many more substances. There are literally thousands of medical applications from packaging to spinal implants. Based on this information, your supplier should be familiar with the types of polymers and composites you need machined. They should additionally know the best machining process for your application. That’s why design conception is crucial as the first step.
1. Design requirements and constraints
First and foremost, what is the function of the polymer for the device?
Your machine shop should be asking you in depth questions about your medical application. Questions to consider include:
- Should the material be biocompatible?
- Is the product for single use?
- Will the component undergo sterilization? If so, which method?
- Does color and aesthetics matter in the machining process?
- Is UV resistance needed?
- What tolerances must be met for temperature, wear, impact, etc?
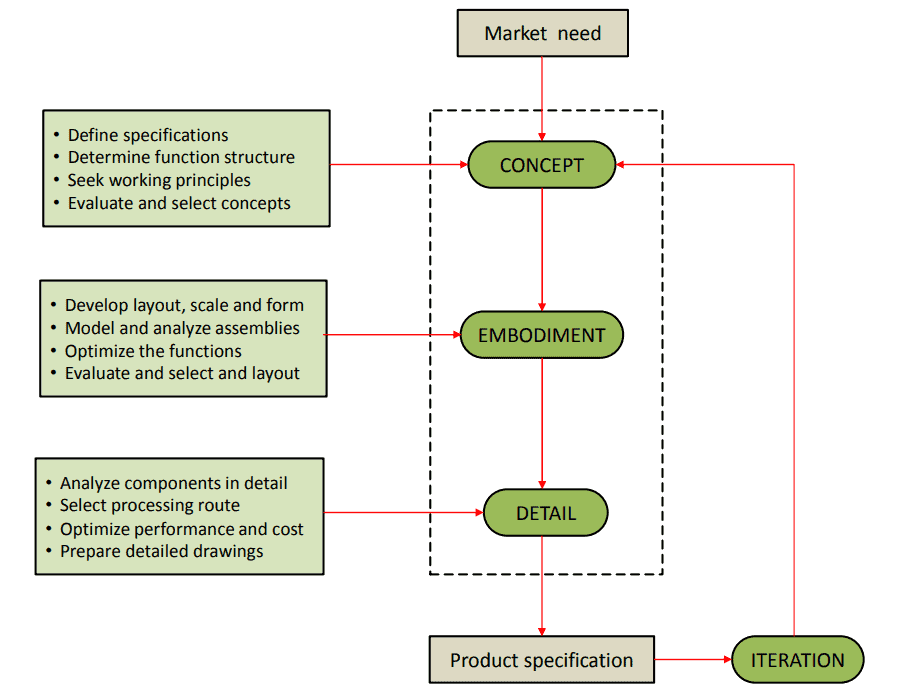
The following material selection flow-chart displays the overall process of developing a medical grade plastic part or device:
2. Industry Standards
What industry standards and regulations control the production of the material?
At AIP, our plastics are processed with strict hygienic procedures to ensure the highest level of sanitation. Make sure that your machining company is compliant and/or registered with the appropriate regulatory organizations. Some common medical certifications include the following:
- ISO 10993
- ISO 13485:2016
- FDA Registered
3. Biocompatibility
How long does the component need to be in contact with the human body or tissue?
There are three categories of contact duration if a component is subject to body tissue or fluid:
- Short-term contact (Less than 24 hours)
- Medium term contact (Between 1-30 days)
- Long-term or Permanent contact (Greater than 30 days)
4. Sterilization/Cleanliness
Will the device need to be resistant to chemicals or undergo multiple sterilizations?
Whether it’s a feeding tube, a drug delivery device or a surgical implant, the polymer material must be able to withstand chemical degradation and multiple sterilizations.
The most common sterilization methods include:
- Radiation (gamma/e-beam)
- Chemical (ETO)
- Autoclave (steam)
Chemicals to consider for contact with the device could be:
- Intravenous medications
- Blood/Fluids/human tissue
- Hospital cleaners – bleach, isopropyl alcohol, peroxides
5. Polymer Characteristics
What mechanical properties does the polymer need to fulfill?
Selecting a plastic material is based on a number of traditional material requirements such as strength, stiffness or impact resistance. Engineered thermoplastics like PC, PEEK, PPSU, POM, show excellent mechanical properties at low and high temperatures. These properties are required for a variety of climate conditions, including during transportation, where the influence of temperature on drop impact may result in different outcomes for device integrity.
Your machinist should be able to give you details about all the plastics in their portfolio such as:
- High wear resistance
- Tensile strength
- Temperature resistance
- Corrosion resistance
- Durability
- Dimensional stability
6. Aesthetics
Does the end product need to be a certain color or have certain qualities?
For instance, if it is a prosthetic for a foot, the polymer needs to be machined a certain shade to match skin tone.
7. Other Material Selection Factors
Anything else?
Other factors to consider and discuss with your machinist include additives for increasing the performance of the polymer, manufacturing process as well as the cost. Take into account the following:
- Radiopacity
- Conductive
- Lubrication
- Manufacturing feasibility
- What manufacturing processes are you using and why?
- Technical performance
- Can we make this product with the material, and can we make it well?
- Economics
- If we can make it, can we make it for a reasonable cost?
At AIP, we are unrivaled experts in medical grade plastic machining.
Talk to our team about how to bring your project from concept to completion.
Contact Us |